Background
Chronic lymphocytic leukemia (CLL) is the most common leukemia in the Western world, with patients facing years of monitoring and treatment. The CLL Advocates Network (CLLAN) was interested in exploring the experience of patients and support organisations to access of treatment and care. The analysis was based on the results of two surveys: the CLL Patient Advocacy and Support Survey(CLL-PAGS)conducted by CLLAN in 2021, and the Global Leukemia Patient Experience Survey (GPES) conducted in the same year in collaboration with Acute Leukemia Advocates Network and CML Advocates Network. CLL-specific data were extracted from the latter survey.
Methodology
The CLL-PAGSexplored the services provided by organisations for patients and their views on healthcare in their respective countries. The GPES provided data from questions completed by patients on their experience across their CLL journey. The surveys were available in 7 (CLL-PAGS) and 10 (GPES) languages and online only. Global recruitment through patient organisations, email, online forums, newsletter, and social media. Survey design was developed through literature review, patient advisory session and expert panel insights. The fieldwork of theCLL-PAGS was conducted for 12 weeks closing in August 2021. The GPES was open between September and November 2021.
Building on analysis of socio demographics of age and gender, countries were segmented into High income (HIC) and Low-and-middle-income countries (LMIC), as per the Organisation for Economic Co-operation and Development´s (OECD) classification.
Results
57 organisations from 40 countries (19 from LMIC and 38 from HIC) responded to the CLL-PAGS. The GPES received a total of 2,646 responses from 40 countries, 45% of whom (n=1,202 in 30 countries) corresponded to CLL. 1118 patients from HIC and 11 patients from 8 countries responded within the LMIC cohort. 73 respondents did not report a country. Because of the difficulty retrieving significant patient information from LMIC, we believe there's value in the results from our small sample.
Age distribution in the GPES was similar to previous studies, with CLL predominantly in older people. 19 patients were aged 35-44 years with 2% <35 years. There was higher representation in LMIC of younger people (90% 35-64 years). 65% of respondents from HIC were >65 years.
Geographical region demonstrated clear differences with HIC (34%) respondents being asymptomatic at diagnosis and all respondents from LMIC being symptomatic. Of those who sought investigation of symptoms, 36% of HIC and 33% of LMIC were diagnosed after first consult. LMIC compared to HIC had delay with 33% diagnosed within 3 and 33% within 5 or more visits.
There was a difference between LMIC and HIC in treatment start. All respondents from LMIC commenced treatment within two years (42% in HIC) which may be attributed to the symptomatic presentation.
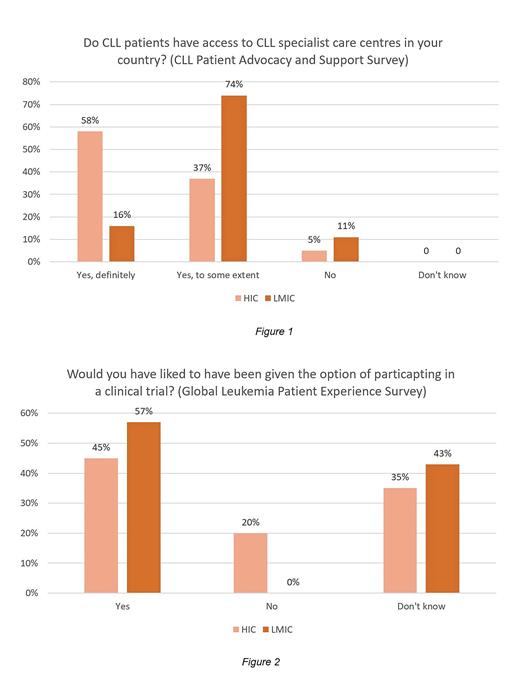
Access to CLL specialist care differed as per figure 1.
Variance in current treatment with 75% of LMIC on chemotherapy, while HIC reported receiving oral targeted therapy (59%) or immunotherapy (21%).
Gaps were shared globally with 52% reporting they had no choice in treatment decision. LMIC were less likely at 60%. Scaled ranking identified only 54% of respondents (30% in LMIC) felt completely involved in treatment decisions.
3/4 of patients globally reported provision of information by treating team about their most recent treatment. This was lower in LMIC (50%). Further divide on patient literacy with 71% of HIC patients and only 40% in LMIC reporting information provision as well understood.
54% reported not being given the option to participate in trials. This was higher in LMIC (70%) than HIC (53%). Preference of being offered a clinical trial is shown in figure 2. Of those who had participated in a trial, 86% found this a positive experience, indicating an opportunity for the research community.
Conclusion
Overall, patients would like more choice in treatment decisions, increased access to clinical trials and better information.
The survey responses and wider literature around CLL suggest that education for patients and HCPs around clinical trials would be beneficial, as would new methods of communicating and engaging with LMIC patients.
There is opportunity to improve partnerships between patient organisations and treating teams to address the gaps. The emerging trends from the surveys merit further research.
Disclosures
Huntley:Astra Zeneca: Other: Funding to affiliated organisation ; AbbVie: Other: Funding to affiliated organisation; Gilead: Other: Funding to affiliated organisation; Janssen: Other: Funding to affiliated organisation; Leukemia & Lymphoma Society (LLS): Other: Funding to affiliated organisation; Lilly: Other: Funding to affiliated organisation; Loxo Oncology: Other: Funding to affiliated organisation; BeiGene: Other: Funding to affiliated organisation. Koffman:BeiGene: Current equity holder in publicly-traded company, Other: Funding to affiliated organisation; AbbVie: Current equity holder in publicly-traded company, Other: Funding to affiliated organisation; Gilead: Current equity holder in publicly-traded company, Other: Funding to affiliated organisation; Janssen: Other: Funding to affiliated organisation; Leukemia & Lymphoma Society (LLS): Other: Funding to affiliated organisation; Lilly: Other: Funding to affiliated organisation; Loxo Oncology: Other: Funding to affiliated organisation; Astra Zeneca: Current equity holder in publicly-traded company, Other: Funding to affiliated organisation ; Bristol Myer Squibb: Current equity holder in publicly-traded company, Honoraria; Johnson & Johnson: Current equity holder in publicly-traded company, Honoraria; MEI Pharma: Current equity holder in publicly-traded company; Merck: Current equity holder in publicly-traded company; Miragen Therapeutics: Current equity holder in publicly-traded company; Novartis: Membership on an entity's Board of Directors or advisory committees; Portola Pharma: Current equity holder in publicly-traded company; Regeneron: Current equity holder in publicly-traded company; Sunesis Pharmaceuticals: Current equity holder in publicly-traded company; TG Therapeutics: Current equity holder in publicly-traded company. Rynne:Astra Zeneca: Other: Funding to affiliated organisation ; AbbVie: Other: Funding to affiliated organisation; Gilead: Other: Funding to affiliated organisation; Janssen: Other: Funding to affiliated organisation; Leukemia & Lymphoma Society (LLS): Other: Funding to affiliated organisation; Lilly: Other: Funding to affiliated organisation; Loxo Oncology: Other: Funding to affiliated organisation; BeiGene: Other: Funding to affiliated organisation. Aumont:Astra Zeneca: Other: Funding to affiliated organisation ; AbbVie: Other: Funding to affiliated organisation; Gilead: Other: Funding to affiliated organisation; Janssen: Other: Funding to affiliated organisation; Leukemia & Lymphoma Society (LLS): Other: Funding to affiliated organisation; Lilly: Other: Funding to affiliated organisation; Loxo Oncology: Other: Funding to affiliated organisation; BeiGene: Other: Funding to affiliated organisation. Bombaci:CLL Advocates Network: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Funding to affiliated organisation; AIL: Membership on an entity's Board of Directors or advisory committees, Other: Funding to affiliated organisation; MPN Advocates Network: Membership on an entity's Board of Directors or advisory committees, Other: Funding to affiliated organisation; CML Advocates Network: Membership on an entity's Board of Directors or advisory committees, Other: Funding to affiliated organisation; Novartis: Consultancy, Other: Funding to affiliated organisation. Schroeter:Astra Zeneca: Other: Funding to affiliated organisation ; AbbVie: Other: Funding to affiliated organisation; Gilead: Other: Funding to affiliated organisation; Janssen: Other: Funding to affiliated organisation; Leukemia & Lymphoma Society (LLS): Other: Funding to affiliated organisation; Lilly: Other: Funding to affiliated organisation; Loxo Oncology: Other: Funding to affiliated organisation; BeiGene: Other: Funding to affiliated organisation. York:Astra Zeneca: Other: Funding to affiliated organisation ; AbbVie: Other: Funding to affiliated organisation; Gilead: Other: Funding to affiliated organisation; Janssen: Other: Funding to affiliated organisation; Leukemia & Lymphoma Society (LLS): Other: Funding to affiliated organisation; Lilly: Other: Funding to affiliated organisation; Loxo Oncology: Other: Funding to affiliated organisation; BeiGene: Membership on an entity's Board of Directors or advisory committees, Other: Funding to affiliated organisation.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal